the neurotoxicity of Mercury and its illegal uses the neurotoxicity of Mercury and its illegal uses and links
😈💩👎
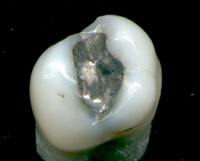
No amount of neurotoxic Mercury(1) is ok in a living body! as stated and agreed upon by 140 countries agreed in the Minamata Convention on Mercury by the United Nations Environment Programme (UNEP) to prevent emissions.[125] The convention was signed on 10 October 2013.[126] That means they know full well that the industry around mercury use such as distribution networks IE dentists and manufacturers and legislators, those who allow their illegal trade and use thereof are fully in breach of the Minamata Convention and deliberately and willfully broke the treaty and acts therein as they already knew that mercury should not be used in fillings for teeth or vaccines or anything else and have continued to do so since that time...I have had all my mercury fillings out, and you should Get them out(2) of your teeth too, but do it with a properly trained hazmat biological dentist as mercury is a class 1 toxic hazardous material(3) and cannot legally be put in with the normal dentistry biological waste because of its toxicity, yet they claim it's perfectly safe to have in your mouth 24/7 even though they know that due to the warmth provided by the human body it releases a constant stream of mercury vapour in your mouth and is ingested by the body through the lungs and stomach lining intestines and all the way down to the colon from a effect called "smoking tooth"(4) (see image 1) which although it just sounds like you may have a staining of the teeth it is in fact quite a different problem and a serious one at that "Smoking Tooth" Evidence(5)
and cause a whole string of diseases and medial conditions that are toxic to life How Mercury Amalgams Are Hurting You(6) from allegedly modern and current applications of mercury(7)
image 1
see links
(1)Mercury
Mercury is a chemical element with the symbol Hg and atomic number 80. It is commonly known as quicksilver and was formerly named hydrargyrum (/haɪˈdrɑːrdʒərəm/ hy-DRAR-jər-əm).[4] A heavy, silvery d-block element, mercury is the only metallic element that is liquid at standard conditions for temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature.
Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is obtained by grinding natural cinnabar or synthetic mercuric sulfide.
Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps and other devices, though concerns about the element's toxicity have led to mercury thermometers and sphygmomanometers being largely phased out in clinical environments in favor of alternatives such as alcohol- or galinstan-filled glass thermometers and thermistor- or infrared-based electronic instruments. Likewise, mechanical pressure gauges and electronic strain gauge sensors have replaced mercury sphygmomanometers.
Mercury remains in use in scientific research applications and in amalgam for dental restoration in some locales. It is also used in fluorescent lighting. Electricity passed through mercury vapor in a fluorescent lamp produces short-wave ultraviolet light, which then causes the phosphor in the tube to fluoresce, making visible light.
Mercury poisoning can result from exposure to water-soluble forms of mercury (such as mercuric chloride or methylmercury), by inhalation of mercury vapor, or by ingesting any form of mercury.
(2) get them out
Mercury Filling Removal
Many modern dental surgeries use “white fillings” instead (no mercury). However, this still leaves the old fillings that have been there in some cases for several decades. And the “official position” on this mercury is that you shouldn’t remove your fillings because replacing them will only further your exposure to the mercury vapors…
There are surgeries that specialize in mercury filling removal and they use a ventilator to draw the mercury out of the mouth and minimize the amount that is reabsorbed into the body. And you know what they do with the material they remove? It has to be disposed of as toxic waste, not regular trash. Yes, we have toxic waste in our bodies. Like many others, I was even given mercury fillings in the 1980s not because I had cavities, but because of “natural grooves” in my teeth that the dentists decided were best filled. Really, they were just lining their pockets… at the expense of our health.
(3)MERCURY material safety data sheet
MERCURY
MSDS Number: M1599 --- Effective Date: 11/17/99
1. Product Identification
Synonyms: Quicksilver; hydrargyrum; Liquid Silver
CAS No.: 7439-97-6
Molecular Weight: 200.59
Chemical Formula: Hg
Product Codes:
J.T. Baker: 2564, 2567, 2569, 2572
Mallinckrodt: 1278, 1280, 1288
2. Composition/Information on Ingredients
Ingredient CAS No Percent Hazardous
--------------------------------------- ------------ ------- ---------
Mercury 7439-97-6 90 - 100% Yes
3. Hazards Identification
Emergency Overview
--------------------------
DANGER! CORROSIVE. CAUSES BURNS TO SKIN, EYES, AND RESPIRATORY TRACT. MAY BE FATAL IF SWALLOWED OR INHALED. HARMFUL IF ABSORBED THROUGH SKIN. AFFECTS THE KIDNEYS AND CENTRAL NERVOUS SYSTEM. MAY CAUSE ALLERGIC SKIN REACTION.
J.T. Baker SAF-T-DATA(tm) Ratings (Provided here for your convenience)
-----------------------------------------------------------------------------------------------------------
Health Rating: 4 - Extreme (Poison)
Flammability Rating: 0 - None
Reactivity Rating: 1 - Slight
Contact Rating: 3 - Severe (Life)
Lab Protective Equip: GOGGLES; LAB COAT; VENT HOOD; PROPER GLOVES
Storage Color Code: Blue (Health)
-----------------------------------------------------------------------------------------------------------
Potential Health Effects
----------------------------------
Inhalation:
Mercury vapor is highly toxic via this route. Causes severe respiratory tract damage. Symptoms include sore throat, coughing, pain, tightness in chest, breathing difficulties, shortness of breath, headache, muscle weakness, anorexia, gastrointestinal disturbance, ringing in the ear, liver changes, fever, bronchitis and pneumonitis. Can be absorbed through inhalation with symptoms similar to ingestion.
Ingestion:
May cause burning of the mouth and pharynx, abdominal pain, vomiting, corrosive ulceration, bloody diarrhea. May be followed by a rapid and weak pulse, shallow breathing, paleness, exhaustion, tremors and collapse. Delayed death may occur from renal failure. Gastrointenstinal uptake of mercury is less than 5% but its ability to penetrate tissues presents some hazard. Initial symptoms may be thirst, possible abdominal discomfort.
Skin Contact:
Causes irritaton and burns to skin. Symptoms include redness and pain. May cause skin allergy and sensitization. Can be absorbed through the skin with symptoms to parallel ingestion.
Eye Contact:
Causes irritation and burns to eyes. Symptoms include redness, pain, blurred vision; may cause serious and permanent eye damage.
Chronic Exposure:
Chronic exposure through any route can produce central nervous system damage. May cause muscle tremors, personality and behavior changes, memory loss, metallic taste, loosening of the teeth, digestive disorders, skin rashes, brain damage and kidney damage. Can cause skin allergies and accumulate in the body. Repeated skin contact can cause the skin to turn gray in color. A suspected reproductive hazard; may damage the developing fetus and decrease fertility in males and females.
Aggravation of Pre-existing Conditions:
Persons with nervous disorders, or impaired kidney or respiratory function, or a history of allergies or a known sensitization to mercury may be more susceptible to the effects of the substance.
4. First Aid Measures
Inhalation:
Remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention immediately.
Ingestion:
Induce vomiting immediately as directed by medical personnel. Never give anything by mouth to an unconscious person. Get medical attention immediately.
Skin Contact:
Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical attention immediately. Wash clothing before reuse. Thoroughly clean shoes before reuse.
Eye Contact:
Immediately flush eyes with plenty of water for at least 15 minutes, lifting lower and upper eyelids occasionally. Get medical attention immediately.
5. Fire Fighting Measures
Fire:
Not considered to be a fire hazard.
Explosion:
Not considered to be an explosion hazard.
Fire Extinguishing Media:
Use any means suitable for extinguishing surrounding fire. Do not allow water runoff to enter sewers or waterways.
Special Information:
In the event of a fire, wear full protective clothing and NIOSH-approved self-contained breathing apparatus with full facepiece operated in the pressure demand or other positive pressure mode. Undergoes hazardous reactions in the presence of heat and sparks or ignition. Smoke may contain toxic mercury or mercuric oxide. Smoke may contain toxic mercury or mercuric oxide.
6. Accidental Release Measures
Ventilate area of leak or spill. Clean-up personnel require protective clothing and respiratory protection from vapor. Spills: Pick up and place in a suitable container for reclamation or disposal in a method that does not generate misting. Sprinkle area with sulfur or calcium polysulfide to suppress mercury. Do not flush to sewer. US Regulations (CERCLA) require reporting spills and releases to soil, water and air in excess of reportable quantities. The toll free number for the US Coast Guard National Response Center is (800) 424-8802.
J. T. Baker CINNASORB(R) and RESISORB(R) are recommended for spills of this product.
7. Handling and Storage
Keep in a tightly closed container, stored in a cool, dry, ventilated area. Protect against physical damage. Isolate from any source of heat or ignition. Do not use or store on porous work surfaces (wood, unsealed concrete, etc.). Follow strict hygiene practices. Containers of this material may be hazardous when empty since they retain product residues (vapors, liquid); observe all warnings and precautions listed for the product.
8. Exposure Controls/Personal Protection
Airborne Exposure Limits:
- OSHA Acceptable Ceiling Concentration:
mercury and mercury compounds: 0.1 mg/m3 (TWA), skin
- ACGIH Threshold Limit Value (TLV):
inorganic and metallic mercury, as Hg: 0.025 mg/m3 (TWA) skin, A4 Not classifiable as a human carcinogen.
- ACGIH Biological Exposure Indices:
total inorganic mercury in urine (preshift): 35 ug/g creatinine;
total inorganic mercury in blood (end of shift): 15 ug/l.
Ventilation System:
A system of local and/or general exhaust is recommended to keep employee exposures below the Airborne Exposure Limits. Local exhaust ventilation is generally preferred because it can control the emissions of the contaminant at its source, preventing dispersion of it into the general work area. Please refer to the ACGIH document, Industrial Ventilation, A Manual of Recommended Practices, most recent edition, for details.
Personal Respirators (NIOSH Approved):
If the exposure limit is exceeded, a half-face respirator with a mercury vapor or chlorine gas cartridge may be worn for up to ten times the exposure limit or the maximum use concentration specified by the appropriate regulatory agency or respirator supplier, whichever is lowest. A full-face piece respirator with a mercury vapor or chlorine gas cartridge may be worn up to 50 times the exposure limit, or the maximum use concentraiton specified by the appropriate regulatory agency or respirator supplier, whichever is lowest. For emergencies or instances where the exposure levels are not known, use a full-face piece positive-pressure, air-supplied respirator. WARNING: Air-purifying respirators do not protect workers in oxygen-deficient atmospheres.
Skin Protection:
Wear impervious protective clothing, including boots, gloves, lab coat, apron or coveralls, as appropriate, to prevent skin contact.
Eye Protection:
Use chemical safety goggles and/or a full face shield where splashing is possible. Maintain eye wash fountain and quick-drench facilities in work area.
9. Physical and Chemical Properties
Appearance:
Silver-white, heavy, mobile, liquid metal.
Odor:
Odorless.
Solubility:
Insoluble in water.
Density:
13.55
pH:
No information found.
% Volatiles by volume @ 21C (70F):
100
Boiling Point:
356.7C (675F)
Melting Point:
-38.87C (-38F)
Vapor Density (Air=1):
7.0
Vapor Pressure (mm Hg):
0.0018 @ 25C (77F)
Evaporation Rate (BuAc=1):
4
10. Stability and Reactivity
Stability:
Stable under ordinary conditions of use and storage.
Hazardous Decomposition Products:
At high temperatures, vaporizes to form extremely toxic fumes.
Hazardous Polymerization:
Will not occur.
Incompatibilities:
Acetylenes, ammonia, ethylene oxide, chlorine dioxide, azides, metal oxides, methyl silane, lithium, rubidium, oxygen, strong oxidants, metal carbonyls.
Conditions to Avoid:
Heat, flames, ignition sources, metal surfaces and incompatibles.
11. Toxicological Information
Toxicological Data:
Investigated as a tumorigen, mutagen, reproductive effector.
Reproductive Toxicity:
All forms of mercury can cross the placenta to the fetus, but most of what is known has been learned from experimental animals. See Chronic Health Hazards.
Carcinogenicity:
EPA / IRIS classification: Group D1 - Not classifiable as a human carcinogen.
--------\Cancer Lists\------------------------------------------------------
---NTP Carcinogen---
Ingredient Known Anticipated IARC Category
------------------------------------ ----- ----------- -------------
Mercury (7439-97-6) No No 3
12. Ecological Information
Environmental Fate:
This material has an experimentally-determined bioconcentration factor (BCF) of greater than 100. This material is expected to significantly bioaccumulate.
Environmental Toxicity:
This material is expected to be toxic to aquatic life. The LC50/96-hour values for fish are less than 1 mg/l.
13. Disposal Considerations
Whatever cannot be saved for recovery or recycling should be handled as hazardous waste and sent to a RCRA approved waste facility. Processing, use or contamination of this product may change the waste management options. State and local disposal regulations may differ from federal disposal regulations. Dispose of container and unused contents in accordance with federal, state and local requirements.
14. Transport Information
Domestic (Land, D.O.T.)
-----------------------
Proper Shipping Name: RQ, MERCURY
Hazard Class: 8
UN/NA: UN2809
Packing Group: III
Information reported for product/size: 2.5KG
International (Water, I.M.O.)
-----------------------------
Proper Shipping Name: MERCURY
Hazard Class: 8
UN/NA: UN2809
Packing Group: III
Information reported for product/size: 2.5KG
International (Air, I.C.A.O.)
-----------------------------
Proper Shipping Name: MERCURY
Hazard Class: 8
UN/NA: UN2809
Packing Group: III
Information reported for product/size: 2.5KG
15. Regulatory Information
--------\Chemical Inventory Status - Part 1\---------------------------------
Ingredient TSCA EC Japan Australia
----------------------------------------------- ---- --- ----- ---------
Mercury (7439-97-6) Yes Yes No Yes
--------\Chemical Inventory Status - Part 2\---------------------------------
--Canada--
Ingredient Korea DSL NDSL Phil.
----------------------------------------------- ----- --- ---- -----
Mercury (7439-97-6) Yes Yes No Yes
--------\Federal, State & International Regulations - Part 1\----------------
-SARA 302- ------SARA 313------
Ingredient RQ TPQ List Chemical Catg.
----------------------------------------- --- ----- ---- --------------
Mercury (7439-97-6) No No Yes No
--------\Federal, State & International Regulations - Part 2\----------------
-RCRA- -TSCA-
Ingredient CERCLA 261.33 8(d)
----------------------------------------- ------ ------ ------
Mercury (7439-97-6) 1 U151 No
Chemical Weapons Convention: No TSCA 12(b): No CDTA: No
SARA 311/312: Acute: Yes Chronic: Yes Fire: No Pressure: No
Reactivity: No (Pure / Liquid)
WARNING:
THIS PRODUCT CONTAINS A CHEMICAL(S) KNOWN TO THE STATE OF CALIFORNIA TO CAUSE BIRTH DEFECTS OR OTHER REPRODUCTIVE HARM.
Australian Hazchem Code: 2Z
Poison Schedule: S7
WHMIS:
This MSDS has been prepared according to the hazard criteria of the Controlled Products Regulations (CPR) and the MSDS contains all of the information required by the CPR.
16. Other Information
NFPA Ratings: Health: 3 Flammability: 0 Reactivity: 0
Label Hazard Warning:
DANGER! CORROSIVE. CAUSES BURNS TO SKIN, EYES, AND RESPIRATORY TRACT. MAY BE FATAL IF SWALLOWED OR INHALED. HARMFUL IF ABSORBED THROUGH SKIN. AFFECTS THE KIDNEYS AND CENTRAL NERVOUS SYSTEM. MAY CAUSE ALLERGIC SKIN REACTION.
Label Precautions:
Do not get in eyes, on skin, or on clothing.
Do not breathe vapor.
Keep container closed.
Use only with adequate ventilation.
Wash thoroughly after handling.
Label First Aid:
If swallowed, induce vomiting immediately as directed by medical personnel. Never give anything by mouth to an unconscious person. If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. In case of contact, immediately flush eyes or skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. In all cases get medical attention immediately.
Product Use:
Laboratory Reagent.
Revision Information:
No changes.
Disclaimer:
************************************************************************************************
Mallinckrodt Baker, Inc. provides the information contained herein in good faith but makes no representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. Individuals receiving the information must exercise their independent judgment in determining its appropriateness for a particular purpose. MALLINCKRODT BAKER, INC. MAKES NO REPRESENTATIONS OR WARRANTIES, EITHER EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION ANY WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE WITH RESPECT TO THE INFORMATION SET FORTH HEREIN OR THE PRODUCT TO WHICH THE INFORMATION REFERS. ACCORDINGLY, MALLINCKRODT BAKER, INC. WILL NOT BE RESPONSIBLE FOR DAMAGES RESULTING FROM USE OF OR RELIANCE UPON THIS INFORMATION.
************************************************************************************************
Prepared by: Strategic Services Division
(4)Smoking Tooth
Mercury gas is odorless, colorless, tasteless and toxic. It can only be seen under a black light. This video of mercury vapor out-gassing from a silver amalgam dental filling has outraged the world since it was first demonstrated at an IAOMT meeting.
(5)Smoking tooth Evidence
How much mercury is released from common amalgam fillings that much of the world has in their mouths? Amalgam dental fillings typically contain a mixture of 50% mercury, mixed in with silver, tin and copper alloys. Typically, dentists give the mercury content of these fillings little to no thought during the placement and the removal of these fillings since the dogmatic consensus is that there is no danger. Nothing could be further from the truth. In this video, a general dentist uses an objective mercury vapor analyzer (as used in industry applications and by the government) to show just how much toxic mercury vapor is emitted from an amalgam sample in quantities that will shock you,
(6)How Mercury Amalgams Are Hurting You
Aside from absorption of toxic mercury through the oral mucosa (the mouth’s membrane), it is most dangerous when inhaled. It’s been established by studies that as you brush your teeth and expose your filling to acidic food, the amalgam releases mercury vapors, which are then absorbed by the body. The most dangerous way mercury reaches the bloodstream and travels through the body is through the lungs. It can affect your nervous, digestive, respiratory, and immune systems, causing multi-organ system failure. Even in low concentrations of mercury, tremors, impaired cognition, and sleep disturbance have all been well-documented adverse effects. [7]
Recently, more and more people are looking at how mercury is causing autoimmune disorders like arthritis, Alzheimer’s, and multiple sclerosis – conditions whose etiology (cause) has been mainly unknown and required further research. A 2014 study found that mercury caused autoantibodies to circulate in the bloodstream. [8] Autoantibodies attack the immune system, bringing rise to autoimmune disorders like arthritis, Sjogren’s syndrome, and multiple sclerosis.
More concerning – did you know that research like this has been published since the early 90’s? In 1994, a study cited mercury as one of the causes of multiple sclerosis. This means that the mere presence of low amounts of mercury in the blood can cause your immune system to turn on itself! Without an actively functioning immune system, we become more prone to disease, specifically infectious disease. [9]
Other Health Effects Of Mercury Fillings
As reported by the Journal of Biomedical Physics & Engineering, [10] mercury toxicity can decrease your output of neurotransmitters, namely:
• Dopamine
• Serotonin
• Norepinephrine
• Acetylcholine
Disrupting these neurotransmitters in the brain can cause serious neurological problems.
The mercury ingredient in “silver” fillings also produces dangers due to the fact the fillings have also been found to release mercury when in contact with a strong electromagnetic field.
According to the same journal, exposure to microwave radiation, most commonly associated with an MRI machine or a cellular phone can lead to an increased leak of mercury from the fillings.
As a further matter, the effects of being exposed to stronger magnetic fields lead to increased level of mercury in the body.
The study went on to link these higher than average levels of mercury in maternal women to an increased chance of having a child with abnormalities.
These disorders incorporate:
• Autism
• Attention Deficit Hyperactivity Disorder (ADHD)
• Along with other disorders
These disorders can couple with autoimmune disorders as well. Who would ever think that getting a simple dental procedure could cause such some serious medical ailments?
Could this enormous medical oversight could be responsible for an array of other illnesses? Only further research will tell.
current applications of mercury(7)
The bulb of a mercury-in-glass thermometer
Mercury is used primarily for the manufacture of industrial chemicals or for electrical and electronic applications. It is used in some thermometers, especially ones which are used to measure high temperatures. A still increasing amount is used as gaseous mercury in fluorescent lamps, while most of the other applications are slowly phased out due to health and safety regulations and is in some applications replaced with less toxic but considerably more expensive Galinstan alloy.[45]
Medicine
See also: Amalgam (dentistry)
Amalgam filling
Mercury and its compounds have been used in medicine, although they are much less common today than they once were, now that the toxic effects of mercury and its compounds are more widely understood. The first edition of the Merck's Manual featured many mercuric compounds[46] such as:
Mercauro
Mercuro-iodo-hemol.
Mercury-ammonium chloride
Mercury Benzoate
Mercuric
Mercury Bichloride (Corrosive Mercuric Chloride, U.S.P.)
Mercury Chloride
Mild Mercury Cyanide
Mercury Succinimide
Mercury Iodide
Red Mercury Biniodide
Mercury Iodide
Yellow Mercury Proto-iodide
Black (Hahnemann), Soluble Mercury Oxide
Red Mercury Oxide
Yellow Mercury Oxide
Mercury Salicylate
Mercury Succinimide
Mercury Imido-succinate
Mercury Sulphate
Basic Mercury Subsulphate; Turpeth Mineral
Mercury Tannate
Mercury-Ammonium Chloride
Mercury is an ingredient in dental amalgams. Thiomersal (called Thimerosal in the United States) is an organic compound used as a preservative in vaccines, though this use is in decline.[47] Thiomersal is metabolized to ethyl mercury. Although it was widely speculated that this mercury-based preservative could cause or trigger autism in children, scientific studies showed no evidence supporting any such link.[48] Nevertheless, thiomersal has been removed from, or reduced to trace amounts in all U.S. vaccines recommended for children 6 years of age and under, with the exception of inactivated influenza vaccine.[49]
Another mercury compound, merbromin (Mercurochrome), is a topical antiseptic used for minor cuts and scrapes that is still in use in some countries.
Mercury in the form of one of its common ores, cinnabar, is used in various traditional medicines, especially in traditional Chinese medicine. Review of its safety has found that cinnabar can lead to significant mercury intoxication when heated, consumed in overdose, or taken long term, and can have adverse effects at therapeutic doses, though effects from therapeutic doses are typically reversible. Although this form of mercury appears to be less toxic than other forms, its use in traditional Chinese medicine has not yet been justified, as the therapeutic basis for the use of cinnabar is not clear.[50]
Today, the use of mercury in medicine has greatly declined in all respects, especially in developed countries. Thermometers and sphygmomanometers containing mercury were invented in the early 18th and late 19th centuries, respectively. In the early 21st century, their use is declining and has been banned in some countries, states and medical institutions. In 2002, the U.S. Senate passed legislation to phase out the sale of non-prescription mercury thermometers. In 2003, Washington and Maine became the first states to ban mercury blood pressure devices.[51] Mercury compounds are found in some over-the-counter drugs, including topical antiseptics, stimulant laxatives, diaper-rash ointment, eye drops, and nasal sprays. The FDA has "inadequate data to establish general recognition of the safety and effectiveness" of the mercury ingredients in these products.[52] Mercury is still used in some diuretics although substitutes now exist for most therapeutic uses.
Production of chlorine and caustic soda
Chlorine is produced from sodium chloride (common salt, NaCl) using electrolysis to separate the metallic sodium from the chlorine gas. Usually the salt is dissolved in water to produce a brine. By-products of any such chloralkali process are hydrogen (H2) and sodium hydroxide (NaOH), which is commonly called caustic soda or lye. By far the largest use of mercury[53][54] in the late 20th century was in the mercury cell process (also called the Castner-Kellner process) where metallic sodium is formed as an amalgam at a cathode made from mercury; this sodium is then reacted with water to produce sodium hydroxide.[55] Many of the industrial mercury releases of the 20th century came from this process, although modern plants claimed to be safe in this regard.[54] After about 1985, all new chloralkali production facilities that were built in the United States used membrane cell or diaphragm cell technologies to produce chlorine.
Laboratory uses
Some medical thermometers, especially those for high temperatures, are filled with mercury; they are gradually disappearing. In the United States, non-prescription sale of mercury fever thermometers has been banned since 2003.[56]
Some transit telescopes use a basin of mercury to form a flat and absolutely horizontal mirror, useful in determining an absolute vertical or perpendicular reference. Concave horizontal parabolic mirrors may be formed by rotating liquid mercury on a disk, the parabolic form of the liquid thus formed reflecting and focusing incident light. Such liquid-mirror telescopes are cheaper than conventional large mirror telescopes by up to a factor of 100, but the mirror cannot be tilted and always points straight up.[57][58][59]
Liquid mercury is a part of popular secondary reference electrode (called the calomel electrode) in electrochemistry as an alternative to the standard hydrogen electrode. The calomel electrode is used to work out the electrode potential of half cells.[60] Last, but not least, the triple point of mercury, −38.8344 °C, is a fixed point used as a temperature standard for the International Temperature Scale (ITS-90).[5]
In polarography both the dropping mercury electrode[61] and the hanging mercury drop electrode[62] use elemental mercury. This use allows a new uncontaminated electrode to be available for each measurement or each new experiment.
Mercury-containing compounds are also of use in the field of structural biology. Mercuric compounds such as mercury(II) chloride or potassium tetraiodomercurate(II) can be added to protein crystals in an effort to create heavy atom derivatives that can be used to solve the phase problem in X-ray crystallography via isomorphous replacement or anomalous scattering methods.
Niche uses
Gaseous mercury is used in mercury-vapor lamps and some "neon sign" type advertising signs and fluorescent lamps. Those low-pressure lamps emit very spectrally narrow lines, which are traditionally used in optical spectroscopy for calibration of spectral position. Commercial calibration lamps are sold for this purpose; reflecting a fluorescent ceiling light into a spectrometer is a common calibration practice.[63] Gaseous mercury is also found in some electron tubes, including ignitrons, thyratrons, and mercury arc rectifiers.[64] It is also used in specialist medical care lamps for skin tanning and disinfection.[65] Gaseous mercury is added to cold cathode argon-filled lamps to increase the ionization and electrical conductivity. An argon-filled lamp without mercury will have dull spots and will fail to light correctly. Lighting containing mercury can be bombarded/oven pumped only once. When added to neon filled tubes the light produced will be inconsistent red/blue spots until the initial burning-in process is completed; eventually it will light a consistent dull off-blue color.[66]
The deep violet glow of a mercury vapor discharge in a germicidal lamp, whose spectrum is rich in invisible ultraviolet radiation.
Skin tanner containing a low-pressure mercury vapor lamp and two infrared lamps, which act both as light source and electrical ballast
Assorted types of fluorescent lamps.
The miniaturized Deep Space Atomic Clock is a linear ion-trap-based mercury ion clock, designed for precise and real-time radio navigation in deep space.
The Deep Space Atomic Clock (DSAC) under development by the Jet Propulsion Laboratory utilises mercury in a linear ion-trap-based clock. The novel use of mercury allows very compact atomic clocks, with low energy requirements, and is therefore ideal for space probes and Mars missions.[67]
Cosmetics
Mercury, as thiomersal, is widely used in the manufacture of mascara. In 2008, Minnesota became the first state in the United States to ban intentionally added mercury in cosmetics, giving it a tougher standard than the federal government.[68]
A study in geometric mean urine mercury concentration identified a previously unrecognized source of exposure (skin care products) to inorganic mercury among New York City residents. Population-based biomonitoring also showed that mercury concentration levels are higher in consumers of seafood and fish meals.[69]
Firearms
Mercury(II) fulminate is a primary explosive which is mainly used as a primer of a cartridge in firearms.
Historic uses
A Single-Pole, Single-Throw (SPST) mercury switch.
Mercury manometer to measure pressure
Many historic applications made use of the peculiar physical properties of mercury, especially as a dense liquid and a liquid metal:
Quantities of liquid mercury ranging from 90 to 600 grams (3.2 to 21.2 oz) have been recovered from elite Maya tombs (100-700AD)[22] or ritual caches at six sites. This mercury may have been used in bowls as mirrors for divinatory purposes. Five of these date to the Classic Period of Maya civilization (c. 250–900) but one example predated this.[70]
In Islamic Spain, it was used for filling decorative pools. Later, the American artist Alexander Calder built a mercury fountain for the Spanish Pavilion at the 1937 World Exhibition in Paris. The fountain is now on display at the Fundació Joan Miró in Barcelona.[71]
Mercury was used inside wobbler lures. Its heavy, liquid form made it useful since the lures made an attractive irregular movement when the mercury moved inside the plug. Such use was stopped due to environmental concerns, but illegal preparation of modern fishing plugs has occurred.
The Fresnel lenses of old lighthouses used to float and rotate in a bath of mercury which acted like a bearing.[72]
Mercury sphygmomanometers (blood pressure meter), barometers, diffusion pumps, coulometers, and many other laboratory instruments. As an opaque liquid with a high density and a nearly linear thermal expansion, it is ideal for this role.[73]
As an electrically conductive liquid, it was used in mercury switches (including home mercury light switches installed prior to 1970), tilt switches used in old fire detectors, and tilt switches in some home thermostats.[74]
Owing to its acoustic properties, mercury was used as the propagation medium in delay line memory devices used in early digital computers of the mid-20th century.
Experimental mercury vapor turbines were installed to increase the efficiency of fossil-fuel electrical power plants.[75] The South Meadow power plant in Hartford, CT employed mercury as its working fluid, in a binary configuration with a secondary water circuit, for a number of years starting in the late 1920s in a drive to improve plant efficiency. Several other plants were built, including the Schiller Station in Portsmouth, NH, which went online in 1950. The idea did not catch on industry-wide due to the weight and toxicity of mercury, as well as the advent of supercritical steam plants in later years.[76][77]
Similarly, liquid mercury was used as a coolant for some nuclear reactors; however, sodium is proposed for reactors cooled with liquid metal, because the high density of mercury requires much more energy to circulate as coolant.[78]
Mercury was a propellant for early ion engines in electric space propulsion systems. Advantages were mercury's high molecular weight, low ionization energy, low dual-ionization energy, high liquid density and liquid storability at room temperature. Disadvantages were concerns regarding environmental impact associated with ground testing and concerns about eventual cooling and condensation of some of the propellant on the spacecraft in long-duration operations. The first spaceflight to use electric propulsion was a mercury-fueled ion thruster developed by NASA Lewis and flown on the Space Electric Rocket Test "SERT-1" spacecraft launched by NASA at its Wallops Flight Facility in 1964. The SERT-1 flight was followed up by the SERT-2 flight in 1970. Mercury and caesium were preferred propellants for ion engines until Hughes Research Laboratory performed studies finding xenon gas to be a suitable replacement. Xenon is now the preferred propellant for ion engines as it has a high molecular weight, little or no reactivity due to its noble gas nature, and has a high liquid density under mild cryogenic storage.[79][80]
Others applications made use of the chemical properties of mercury:
The mercury battery is a non-rechargeable electrochemical battery, a primary cell, that was common in the middle of the 20th century. It was used in a wide variety of applications and was available in various sizes, particularly button sizes. Its constant voltage output and long shelf life gave it a niche use for camera light meters and hearing aids. The mercury cell was effectively banned in most countries in the 1990s due to concerns about the mercury contaminating landfills.[81]
Mercury was used for preserving wood, developing daguerreotypes, silvering mirrors, anti-fouling paints (discontinued in 1990), herbicides (discontinued in 1995), handheld maze games, cleaning, and road leveling devices in cars. Mercury compounds have been used in antiseptics, laxatives, antidepressants, and in antisyphilitics.
It was allegedly used by allied spies to sabotage Luftwaffe planes: a mercury paste was applied to bare aluminium, causing the metal to rapidly corrode; this would cause structural failures.[82]
Chloralkali process: The largest industrial use of mercury during the 20th century was in electrolysis for separating chlorine and sodium from brine; mercury being the anode of the Castner-Kellner process. The chlorine was used for bleaching paper (hence the location of many of these plants near paper mills) while the sodium was used to make sodium hydroxide for soaps and other cleaning products. This usage has largely been discontinued, replaced with other technologies that utilize membrane cells.[83]
As electrodes in some types of electrolysis, batteries (mercury cells), sodium hydroxide and chlorine production, handheld games, catalysts, insecticides.
From the mid-18th to the mid-19th centuries, a process called "carroting" was used in the making of felt hats. Animal skins were rinsed in an orange solution (the term "carroting" arose from this color) of the mercury compound mercuric nitrate, Hg(NO3)2·2H2O.[86] This process separated the fur from the pelt and matted it together. This solution and the vapors it produced were highly toxic. The United States Public Health Service banned the use of mercury in the felt industry in December 1941. The psychological symptoms associated with mercury poisoning inspired the phrase "mad as a hatter". Lewis Carroll's "Mad Hatter" in his book Alice's Adventures in Wonderland was a play on words based on the older phrase, but the character himself does not exhibit symptoms of mercury poisoning.[87]
Gold and silver mining. Historically, mercury was used extensively in hydraulic gold mining in order to help the gold to sink through the flowing water-gravel mixture. Thin gold particles may form mercury-gold amalgam and therefore increase the gold recovery rates.[5] Large-scale use of mercury stopped in the 1960s. However, mercury is still used in small scale, often clandestine, gold prospecting. It is estimated that 45,000 metric tons of mercury used in California for placer mining have not been recovered.[88] Mercury was also used in silver mining.[89]
Historic medicinal uses
Mercury(I) chloride (also known as calomel or mercurous chloride) has been used in traditional medicine as a diuretic, topical disinfectant, and laxative. Mercury(II) chloride (also known as mercuric chloride or corrosive sublimate) was once used to treat syphilis (along with other mercury compounds), although it is so toxic that sometimes the symptoms of its toxicity were confused with those of the syphilis it was believed to treat.[90] It is also used as a disinfectant. Blue mass, a pill or syrup in which mercury is the main ingredient, was prescribed throughout the 19th century for numerous conditions including constipation, depression, child-bearing and toothaches.[91] In the early 20th century, mercury was administered to children yearly as a laxative and dewormer, and it was used in teething powders for infants. The mercury-containing organohalide merbromin (sometimes sold as Mercurochrome) is still widely used but has been banned in some countries such as the U.S.[92]
Toxicity and safety
See also: Mercury poisoning and Mercury cycle
Mercury (element)Hazards
GHS Signal word Danger
GHS hazard statements H330, H360D, H372, H410
GHS precautionary statements P201, P260, P273, P280, P304, P340, P310, P308, P313, P391, P403, P233[93]
Mercury and most of its compounds are extremely toxic and must be handled with care; in cases of spills involving mercury (such as from certain thermometers or fluorescent light bulbs), specific cleaning procedures are used to avoid exposure and contain the spill.[94] Protocols call for physically merging smaller droplets on hard surfaces, combining them into a single larger pool for easier removal with an eyedropper, or for gently pushing the spill into a disposable container. Vacuum cleaners and brooms cause greater dispersal of the mercury and should not be used. Afterwards, fine sulfur, zinc, or some other powder that readily forms an amalgam (alloy) with mercury at ordinary temperatures is sprinkled over the area before itself being collected and properly disposed of. Cleaning porous surfaces and clothing is not effective at removing all traces of mercury and it is therefore advised to discard these kinds of items should they be exposed to a mercury spill.
Mercury can be absorbed through the skin and mucous membranes and mercury vapors can be inhaled, so containers of mercury are securely sealed to avoid spills and evaporation. Heating of mercury, or of compounds of mercury that may decompose when heated, should be carried out with adequate ventilation in order to minimize exposure to mercury vapor. The most toxic forms of mercury are its organic compounds, such as dimethylmercury and methylmercury. Mercury can cause both chronic and acute poisoning.
Releases in the environment
Amount of atmospheric mercury deposited at Wyoming's Upper Fremont Glacier over the last 270 years
Preindustrial deposition rates of mercury from the atmosphere may be about 4 ng /(1 L of ice deposit). Although that can be considered a natural level of exposure, regional or global sources have significant effects. Volcanic eruptions can increase the atmospheric source by 4–6 times.[95]
Natural sources, such as volcanoes, are responsible for approximately half of atmospheric mercury emissions. The human-generated half can be divided into the following estimated percentages:[96][97][98]
65% from stationary combustion, of which coal-fired power plants are the largest aggregate source (40% of U.S. mercury emissions in 1999). This includes power plants fueled with gas where the mercury has not been removed. Emissions from coal combustion are between one and two orders of magnitude higher than emissions from oil combustion, depending on the country.[96]
11% from gold production. The three largest point sources for mercury emissions in the U.S. are the three largest gold mines. Hydrogeochemical release of mercury from gold-mine tailings has been accounted as a significant source of atmospheric mercury in eastern Canada.[99]
6.8% from non-ferrous metal production, typically smelters.
6.4% from cement production.
3.0% from waste disposal, including municipal and hazardous waste, crematoria, and sewage sludge incineration.
3.0% from caustic soda production.
1.1% from mercury production, mainly for batteries.
2.0% from other sources.
The above percentages are estimates of the global human-caused mercury emissions in 2000, excluding biomass burning, an important source in some regions.[96]
Recent atmospheric mercury contamination in outdoor urban air was measured at 0.01–0.02 µg/m3. A 2001 study measured mercury levels in 12 indoor sites chosen to represent a cross-section of building types, locations and ages in the New York area. This study found mercury concentrations significantly elevated over outdoor concentrations, at a range of 0.0065 – 0.523 μg/m3. The average was 0.069 μg/m3.[100]
Artificial lakes may be contaminated with mercury due to the absorption by the water of mercury from submerged trees and soil. For example, Williston Lake in northern British Columbia, created by the damming of the Peace River in 1968, is still sufficiently contaminated with mercury that it is inadvisable to consume fish from the lake.[101][102]
Mercury also enters into the environment through the improper disposal (e.g., land filling, incineration) of certain products. Products containing mercury include: auto parts, batteries, fluorescent bulbs, medical products, thermometers, and thermostats.[103] Due to health concerns (see below), toxics use reduction efforts are cutting back or eliminating mercury in such products. For example, the amount of mercury sold in thermostats in the United States decreased from 14.5 tons in 2004 to 3.9 tons in 2007.[104]
Most thermometers now use pigmented alcohol instead of mercury, and galinstan alloy thermometers are also an option. Mercury thermometers are still occasionally used in the medical field because they are more accurate than alcohol thermometers, though both are commonly being replaced by electronic thermometers and less commonly by galinstan thermometers. Mercury thermometers are still widely used for certain scientific applications because of their greater accuracy and working range.
Historically, one of the largest releases was from the Colex plant, a lithium-isotope separation plant at Oak Ridge, Tennessee. The plant operated in the 1950s and 1960s. Records are incomplete and unclear, but government commissions have estimated that some two million pounds of mercury are unaccounted for.[105]
A serious industrial disaster was the dumping of mercury compounds into Minamata Bay, Japan. It is estimated that over 3,000 people suffered various deformities, severe mercury poisoning symptoms or death from what became known as Minamata disease.[106][107]
The tobacco plant readily absorbs and accumulates heavy metals such as mercury from the surrounding soil into its leaves. These are subsequently inhaled during tobacco smoking.[108] While mercury is a constituent of tobacco smoke,[109] studies have largely failed to discover a significant correlation between smoking and Hg uptake by humans compared to sources such as occupational exposure, fish consumption, and amalgam tooth fillings.[110]
Sediment contamination
Sediments within large urban-industrial estuaries act as an important sink for point source and diffuse mercury pollution within catchments.[111] A 2015 study of foreshore sediments from the Thames estuary measured total mercury at 0.01 to 12.07 mg/kg with mean of 2.10 mg/kg and median of 0.85 mg/kg (n=351).[111] The highest mercury concentrations were shown to occur in and around the city of London in association with fine grain muds and high total organic carbon content.[111] The strong affinity of mercury for carbon rich sediments has also been observed in salt marsh sediments of the River Mersey mean of 2 mg/kg up to 5 mg/kg.[112] These concentrations are far higher than those shown in salt marsh river creek sediments of New Jersey and mangroves of Southern China which exhibit low mercury concentrations of about 0.2 mg/kg.[113][114]
Occupational exposure
EPA workers clean up residential mercury spill in 2004
Due to the health effects of mercury exposure, industrial and commercial uses are regulated in many countries. The World Health Organization, OSHA, and NIOSH all treat mercury as an occupational hazard, and have established specific occupational exposure limits. Environmental releases and disposal of mercury are regulated in the U.S. primarily by the United States Environmental Protection Agency.
Fish
Main article: Mercury in fish
Fish and shellfish have a natural tendency to concentrate mercury in their bodies, often in the form of methylmercury, a highly toxic organic compound of mercury. Species of fish that are high on the food chain, such as shark, swordfish, king mackerel, bluefin tuna, albacore tuna, and tilefish contain higher concentrations of mercury than others. As mercury and methylmercury are fat soluble, they primarily accumulate in the viscera, although they are also found throughout the muscle tissue.[115] When this fish is consumed by a predator, the mercury level is accumulated. Since fish are less efficient at depurating than accumulating methylmercury, fish-tissue concentrations increase over time. Thus species that are high on the food chain amass body burdens of mercury that can be ten times higher than the species they consume. This process is called biomagnification. Mercury poisoning happened this way in Minamata, Japan, now called Minamata disease.
Cosmetics
Some facial creams contain dangerous levels of mercury. Most contain comparatively non-toxic inorganic mercury, but products containing highly toxic organic mercury have been encountered.[116][117]
Effects and symptoms of mercury poisoning
Main article: Mercury poisoning
Toxic effects include damage to the brain, kidneys and lungs. Mercury poisoning can result in several diseases, including acrodynia (pink disease), Hunter-Russell syndrome, and Minamata disease.
Symptoms typically include sensory impairment (vision, hearing, speech), disturbed sensation and a lack of coordination. The type and degree of symptoms exhibited depend upon the individual toxin, the dose, and the method and duration of exposure. Case–control studies have shown effects such as tremors, impaired cognitive skills, and sleep disturbance in workers with chronic exposure to mercury vapor even at low concentrations in the range 0.7–42 μg/m3.[118][119] A study has shown that acute exposure (4–8 hours) to calculated elemental mercury levels of 1.1 to 44 mg/m3 resulted in chest pain, dyspnea, cough, hemoptysis, impairment of pulmonary function, and evidence of interstitial pneumonitis.[120] Acute exposure to mercury vapor has been shown to result in profound central nervous system effects, including psychotic reactions characterized by delirium, hallucinations, and suicidal tendency. Occupational exposure has resulted in broad-ranging functional disturbance, including erethism, irritability, excitability, excessive shyness, and insomnia. With continuing exposure, a fine tremor develops and may escalate to violent muscular spasms. Tremor initially involves the hands and later spreads to the eyelids, lips, and tongue. Long-term, low-level exposure has been associated with more subtle symptoms of erethism, including fatigue, irritability, loss of memory, vivid dreams and depression.[121][122]
Treatment
Research on the treatment of mercury poisoning is limited. Currently available drugs for acute mercurial poisoning include chelators N-acetyl-D, L-penicillamine (NAP), British Anti-Lewisite (BAL), 2,3-dimercapto-1-propanesulfonic acid (DMPS), and dimercaptosuccinic acid (DMSA). In one small study including 11 construction workers exposed to elemental mercury, patients were treated with DMSA and NAP.[123] Chelation therapy with both drugs resulted in the mobilization of a small fraction of the total estimated body mercury. DMSA was able to increase the excretion of mercury to a greater extent than NAP.[124]
References:
[1] World Health Organization (2011). Replacement of mercury thermometers and sphygmomanometers in healthcare. http://apps.who.int/iris/bitstream/10665/44592/1/9789241548182_eng.pdf
[2] Cabaña-Muñoz, M., et. al. (2015). Increased Zn/Glutathione Levels and Higher Superoxide Dismutase-1 Activity as Biomarkers of Oxidative Stress in Women with Long-Term Dental Amalgam Fillings: Correlation between Mercury/Aluminium Levels (in Hair) and Antioxidant Systems in Plasma. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4468144/
[3] Jones, L., Bunnell, J. & Stillman, J. (2007). A 30-year follow-up of residual effects on New Zealand School Dental Nurses, from occupational mercury exposure. http://www.ncbi.nlm.nih.gov/pubmed/17615119/
[5] Colgate. Dental Amalgam: A Health Risk? http://www.colgate.com/en/us/oc/oral-health/procedures/fillings/article/dental-amalgam-a-health-risk
[6] Consumers for Dental Choice (2014). Measurably Misleading. http://www.toxicteeth.org/measurablymisleading.aspx
[7] Neeti, R. & Singh, R. (2010). Mercury and health care. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2992864/
[8] Motts, J., et. al. (2014). Novel biomarkers of mercury-induced autoimmune dysfunction: a cross-sectional study in Amazonian Brazil. http://www.ncbi.nlm.nih.gov/pubmed/24742722
[9] Siblerud, R. & Kienholz, E. (1994). Evidence that mercury from silver dental fillings may be an etiological factor in multiple sclerosis. http://www.ncbi.nlm.nih.gov/pubmed/8191275
[10] Mortazavi, Gh., M. Haghani, N. Rastegarian, S. Zarei, and S.M.J. Mortazavi. “Increased Release of Mercury from Dental Amalgam Fillings due to Maternal Exposure to Electromagnetic Fields as a Possible Mechanism for the High Rates of Autism in the Offspring: Introducing a Hypothesis”. Shiraz University of Medical Sciences (2016). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4795328/